
First as indicated in the following diagram, these spacer arms vary by length, which can affect the availability of the attached biotin for binding to avidin, streptavidin or NeutrAvidin.Īctive site probes are a class of chemical labeling reagents whose reactive groups are designed to specifically bind (label) particular enzyme active sites. Besides connecting biotin to a chemical group that mediates protein attachment, spacer arms can influence biotinylation and protein detection in three ways. Spacer arms link the biotin molecule to a reactive group that interacts with certain functional groups on the amino acids of the target protein. Variations in these three features account for the many varieties of available reagents and provide the specific properties needed for particular applications. They are composed of the biotinyl group, a spacer arm and a reactive group that is responsible for attachment to target functional groups on proteins.
Chemical methods of biotinylation are most commonly used, and the biotinylation reagents used for this type of labeling share several basic features. In those situations, certain variants of avidin or derivatives of biotin are available, which allow soft-release (elution) binding or cleavable (reversible) labeling.īiotinylation is the process of labeling proteins or nucleotides with biotin molecules and can be performed by enzymatic and chemical means. However, depending on the nature of the application, the very strong binding interaction can be problematic. Together, these features make avidin–biotin strategies ideal for many detection and immobilization applications. Additionally, biotin (244.3 Da) is considerably smaller than enzyme labels and is therefore less likely to interfere with normal protein function. Indeed, this interaction is one of the strongest noncovalent interactions between a protein and ligand. They are different from each other mainly based on the polarity.Biotin is a useful label for protein detection, purification and immobilization because of its extraordinarily strong binding to avidin, streptavidin or Thermo Scientific NeutrAvidin Protein. Amino acids are the building blocks of proteins. The key difference between hydrophobic and hydrophilic amino acids is that the hydrophobic amino acids are nonpolar whereas the hydrophilic amino acids are polar. What is the difference between hydrophobic and hydrophilic amino acids?

Polar side chains contain groups that are either charged at physiological pH or groups that are able to participate in hydrogen bonding. The polar amino acids include: arginine, asparagine, aspartic acid (or aspartate), glutamine, glutamic acid (or glutamate), histidine, lysine, serine, and threonine. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom.Īdditionally, is glutamine polar or nonpolar?
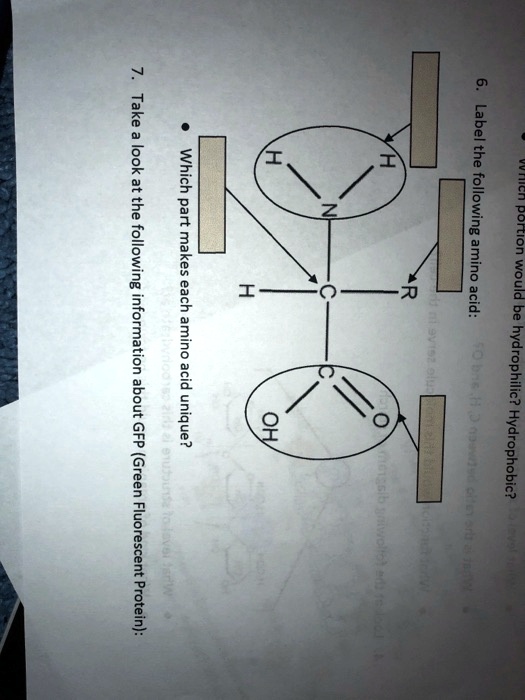
It is the only achiral proteinogenic amino acid. Likewise, is glycine hydrophobic or hydrophilic? Glycine is a colorless, sweet-tasting crystalline solid. Also to know is, is arginine hydrophobic or hydrophilic?Īmino acids are ordered from the most hydrophobic one, Isoleucine (I, on the left hand side) to the most hydrophilic one, Arginine (R, on the right hand side), according to the Kyte-Doolitle scale.


 0 kommentar(er)
0 kommentar(er)
